
How FRUZAQLA works
FRUZAQLA is a novel, selective inhibitor of all 3 VEGF receptors with limited off-target kinase activity, allowing for drug exposure achieving sustained target inhibition1-3*
FRUZAQLA is a small molecule kinase inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, -2, and -31-3
FRUZAQLA is a VEGFR inhibitor that specifically targets and blocks all 3 vascular endothelial growth factor receptors (VEGFR)1-3
- By inhibiting VEGFR-1 and VEGFR-2, FRUZAQLA restricts tumor growth and progression3,6
- By also inhibiting VEGFR-3, FRUZAQLA has the potential to inhibit lymphangiogenesis3,7
*Preclinical activity does not necessarily correlate with clinical outcomes.
Watch this video to learn about FRUZAQLA® (fruquintinib) Mechanism of Action
FRUZAQLA® (fruquintinib) Mechanism of Action
FRUZAQLA is the first and only novel targeted therapy approved for mCRC in more than a decade, for adult patients who have received oxaliplatin- and irinotecan-based regiments, regardless of mutation status.1, 8-11
ViewHide Transcript
With more than 25 years of leadership in oncology, FRUZAQLA emerged from Takeda's commitment to exploring innovative therapies in metastatic colorectal cancer, with an FDA approval for patients with previously treated mCRC.1
FRUZAQLA is the first and only novel targeted therapy approved for mCRC, regardless of mutation status, in more than a decade.1-5
It restricts tumor growth by selectively inhibiting all 3 VEGF receptors.6
This is notable, because the VEGF pathway is primarily responsible for regulating angiogenesis. Angiogenesis is the formation of new blood vessels that enables oxygen and nutrients to reach the tumor, promoting growth. It is a critical step in the development of tumors in CRC.7-9
Angiogenesis is activated when VEGF is released by cancer cells in the tumor microenvironment.9
There are 3 different receptors critical to the growth and progression of CRC: VEGFR-1, VEGFR-2, and VEGFR-3. VEGF binds and activates these receptors…9
…This is why targeting the VEGF pathway has become a mainstay approach for treatment in CRC.10
When VEGF binds to VEGF receptors, downstream signaling pathways are initiated. VEGFR-1 and -2 signaling initiates angiogenesis, which aids in tumor development. VEGFR-3 signaling initiates lymphangiogenesis, which enables tumor metastasis.9,11-13
When VEGF binds to VEGF receptors, it also induces phosphorylation of the kinase domain. This is a chemical reaction (or modification) that activates the VEGF receptor and promotes angiogenesis, helping cancer cells to grow.9,11,14
The PI3K/AkT pathway is one pathway involved in angiogenesis and is activated by VEGFR-1 signaling. This pathway regulates cell migration, invasion, and survival.12
VEGFR-2 signaling also activates the PI3K/AkT pathway, as well as another pathway involved in angiogenesis, the RAS/RAF pathway. The RAS/RAF pathway regulates cell proliferation, permeability, and survival.12,15
Specific components of these 2 pathways (for example, AkT and ERK) are activated by VEGFR-3 signaling, which is involved in lymphangiogenesis (that is, the formation of new lymphatic vessels).16
Targeting the VEGF pathway is a key approach to treating CRC.10,17
FRUZAQLA was designed for the selective inhibition of all 3 VEGF receptors.6
A novel kinase inhibitor, FRUZAQLA restricts tumor growth and progression, with the potential to inhibit lymphangiogenesis.1,18,19
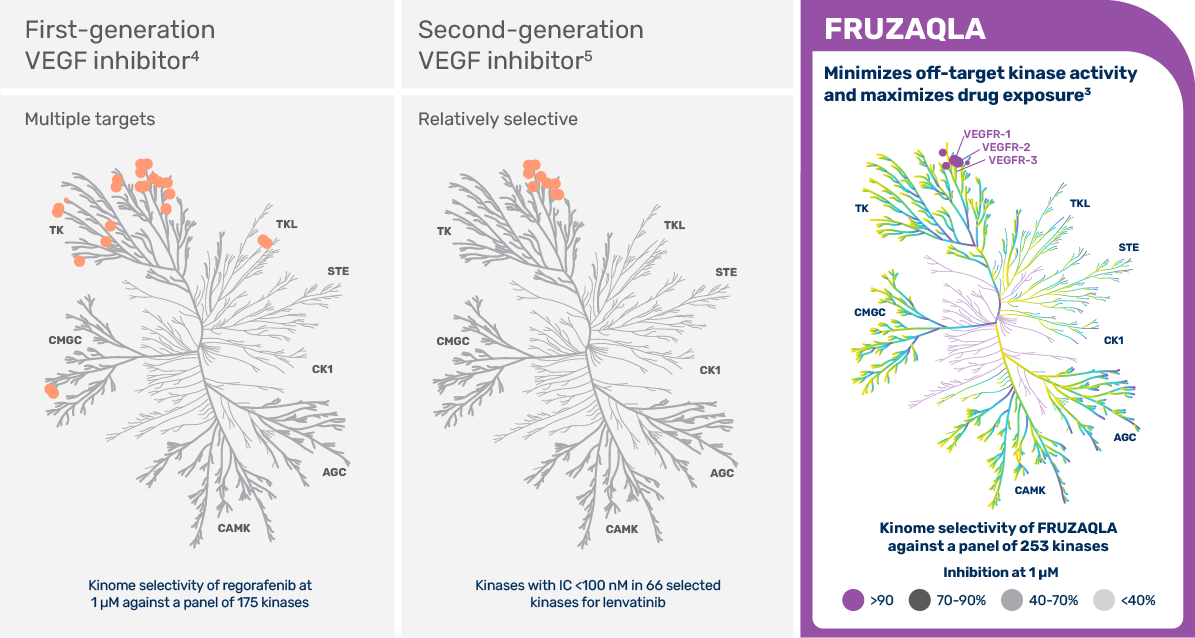
Earlier-generation VEGF inhibitors demonstrate activity at multiple off-target kinases, with limited selectivity for VEGF receptors.20-22
FRUZAQLA is a non-chemotherapy that limits off-target kinase activity, allowing for drug exposure achieving sustained target inhibition.1,6
Most first-generation treatments target the VEGF ligand outside the cell, while FRUZAQLA binds and inhibits VEGFRs inside the cell.6,22
Activation of the intracellular domains involved in angiogenesis and lymphangiogenesis is inhibited by FRUZAQLA.1,6
Phosphorylation is prevented by FRUZAQLA and VEGF receptor signaling is blocked. Targeting VEGFR-1 and -2, FRUZAQLA helps restrict tumor growth and progression. And targeting VEGFR-3 has the potential to inhibit lymphangiogenesis.6,18,19
The novel mechanism of action of FRUZAQLA makes it a treatment worth considering for your appropriate patients with mCRC2,6:
•FRUZAQLA is an alternative to chemotherapy1
•It selectively inhibits all 3 VEGF receptors
•It restricts tumor growth and progression in cells and in vitro6,18
•And FRUZAQLA demonstrates limited off-target kinase activity1,6
FRUZAQLA is indicated for the treatment of adult patients with metastatic colorectal cancer (mCRC) who have been previously treated with fluoropyrimidine‑, oxaliplatin‑, and irinotecan‑based chemotherapy, an anti‑VEGF therapy, and, if RAS wild‑type and medically appropriate, an anti-EGFR therapy.
Important safety information about FRUZAQLA:
WARNINGS AND PRECAUTIONS
Hypertension occurred in 49% of 911 patients with mCRC treated with FRUZAQLA, including Grade 3-4 events in 19%, and hypertensive crisis in three patients (0.3%). Do not initiate FRUZAQLA unless blood pressure is adequately controlled. Monitor blood pressure weekly for the first month and at least monthly thereafter as clinically indicated. Initiate or adjust anti-hypertensive therapy as appropriate. Withhold, reduce dose, or permanently discontinue FRUZAQLA based on severity of hypertension.
Hemorrhagic Events including serious, fatal events can occur with FRUZAQLA. In 911 patients with mCRC treated with FRUZAQLA, 6% of patients experienced gastrointestinal hemorrhage, including 1% with a Grade ≥3 event and 2 patients with fatal hemorrhages. Permanently discontinue FRUZAQLA in patients with severe or life-threatening hemorrhage. Monitor the International Normalized Ratio (INR) levels in patients receiving anticoagulants.
Infections. FRUZAQLA can increase the risk of infections, including fatal infections. In 911 patients with mCRC treated with FRUZAQLA, the most common infections were urinary tract infections (6.8%), upper respiratory tract infections (3.2%) and pneumonia (2.5%); fatal infections included pneumonia (0.4%), sepsis (0.2%), bacterial infection (0.1%), lower respiratory tract infection (0.1%), and septic shock (0.1%). Withhold FRUZAQLA for Grade 3 or 4 infections, or worsening infection of any grade. Resume FRUZAQLA at the same dose when the infection has resolved.
Gastrointestinal Perforation occurred in patients treated with FRUZAQLA. In 911 patients with mCRC treated with FRUZAQLA, 1.3% experienced a Grade ≥3 gastrointestinal perforation, including one fatal event. Permanently discontinue FRUZAQLA in patients who develop gastrointestinal perforation or fistula.
Hepatotoxicity. FRUZAQLA can cause liver injury. In 911 patients with mCRC treated with FRUZAQLA, 48% experienced increased ALT or AST, including Grade ≥3 events in 5%, and fatal events in 0.2% of patients.
Monitor liver function tests (ALT, AST, and bilirubin) before initiation and periodically throughout treatment with FRUZAQLA. Temporarily hold and then reduce or permanently discontinue FRUZAQLA depending on the severity and persistence of hepatotoxicity as manifested by elevated liver function tests.
Proteinuria. FRUZAQLA can cause proteinuria. In 911 patients with mCRC treated with FRUZAQLA, 36% experienced proteinuria and 2.5% of patients experienced Grade ≥3 events. Monitor for proteinuria before initiation and periodically throughout treatment with FRUZAQLA. For proteinuria ≥2g/24 hours, withhold FRUZAQLA until improvement to ≤Grade 1 proteinuria and resume FRUZAQLA at a reduced dose. Discontinue FRUZAQLA in patients who develop nephrotic syndrome.
Palmar-Plantar Erythrodysesthesia (PPE) occurred in 35% of 911 patients treated with FRUZAQLA, including 8% with Grade 3 events. Based on severity of PPE, withhold FRUZAQLA and then resume at the same or reduced dose.
Posterior Reversible Encephalopathy Syndrome (PRES), a syndrome of subcortical vasogenic edema diagnosed by characteristic finding on MRI, occurred in one of 911 patients treated with FRUZAQLA. Perform an evaluation for PRES in any patient presenting with seizures, headache, visual disturbances, confusion, or altered mental function. Discontinue FRUZAQLA in patients who develop PRES.
Impaired Wound Healing. In 911 patients with mCRC treated with FRUZAQLA, 1 patient experienced a Grade 2 event of wound dehiscence. Do not administer FRUZAQLA for at least 2 weeks prior to major surgery. Do not administer FRUZAQLA for at least 2 weeks after major surgery and until adequate wound healing. The safety of resumption of FRUZAQLA after resolution of wound healing complications has not been established.
Arterial Thromboembolic Events. In 911 patients with mCRC treated with FRUZAQLA, 0.8% of patients experienced an arterial thromboembolic event. Initiation of FRUZAQLA in patients with a recent history of thromboembolic events should be carefully considered. In patients who develop arterial thromboembolism, discontinue FRUZAQLA.
Allergic Reactions to FD&C Yellow No. 5 (Tartrazine) and No. 6 (Sunset Yellow FCF). FRUZAQLA 1 mg capsules contain FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. FRUZAQLA 1 mg contains FD&C Yellow No. 6 (sunset yellow FCF), which may cause allergic reactions.
Embryo-Fetal Toxicity. Based on findings in animal studies and its mechanism of action, FRUZAQLA can cause fetal harm when administered to pregnant women. Advise pregnant women of the potential risk to a fetus.
ADVERSE REACTIONS
The most common adverse reactions (incidence ≥20%) following treatment with FRUZAQLA included hypertension, palmar-plantar erythrodysesthesia (hand-foot skin reactions), proteinuria, dysphonia, abdominal pain, diarrhea, and asthenia.
DRUG INTERACTIONS: Avoid concomitant administration of FRUZAQLA with strong or moderate CYP3A inducers.
USE IN SPECIFIC POPULATIONS
Lactation: Advise women not to breastfeed during treatment with FRUZAQLA and for 2 weeks after the last dose.
Females and Males of Reproductive Potential
Pregnancy Testing: Verify pregnancy status of females of reproductive potential prior to initiating FRUZAQLA.
Contraception: Females of childbearing potential and males with female partners of childbearing potential should use effective contraception during treatment and for 2 weeks after the last dose of FRUZAQLA.
Infertility: Advise females of reproductive potential that FRUZAQLA may cause post-implantation loss.
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-844-662-8532 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
